Abstract
Non-severe aplastic anemia (NSAA) or moderate AA is a rare and heterogeneous bone marrow failure disorder characterized by moderate degree of cytopenia that does not meet conventional criteria for severe/very severe AA. Data on clinical workup and therapeutic strategies are largely lacking and the clinical management is chiefly based on individual centers’ expertise. With the aim of deciphering treatment patterns and outcomes of moderate AA, we accrued 238 patients diagnosed with NSAA according to Camitta criteria at 4 tertiary hematologic centers in Italy (Milan and Rome), USA, and UK between 1980-2022. Baseline hematologic features, bone marrow assessment, flow-cytometry (FC) and mutational status of common myeloid drivers were analysed. The different management strategies were registered and categorized as: 1) cyclosporin +/- steroids (CyA), 2) eltrombopag + CyA, 3) eltrombopag single agent, and 4) other. Responses were assessed according to EBMT criteria (complete, CR, if platelets PLT >100 x 10^9/L, hemoglobin Hb >10 g/dL, neutrophils-ANC > 1.5x10^9/L; partial if transfusion independent).
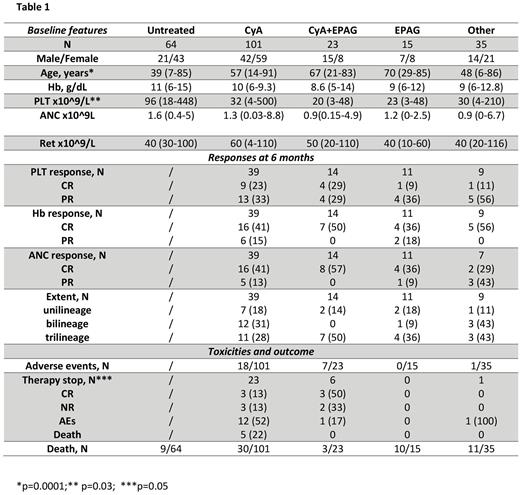
As shown in Table 1, patients’ median age was 53 years, range (6-91). At baseline, 53% of cases presented with thrombocytopenia <50x10^9/L, 43% with moderate anemia (Hb <10 g/dL; 11% <8 g/dL), and 49% with neutropenia (<1.5 x10^9/L) with 23% being transfusion dependent for PLT and 20% for red blood cells (RBC). A PNH clone was present in 40% of cases with a median size of 0.5% (0.1-99) on granulocytes. Bone marrow evaluation showed a median cellularity of 15% (5-30), with reticulin fibrosis (MF-1) in 17/94 (18%) evaluable patients. FC (N=45) revealed a polyclonal T-cell infiltrate in 26 patients (58%), mixed T- and B-cell in 7 (15%), and no infiltrate in the others. Cytogenetics failed in 3 and was abnormal in 7 patients. Targeted panel sequencing (N=100) showed at least one mutation in 16 patients, 2 mutations in 4, and 3 mutations in 2 cases (including 3 mutations in BCOR, 5 in TET2, and 1 each in DNMT3A, CUX1, SRSF2, U2AF1, NRAS, ASXL1, CBL, EZH2, CALR, ETV6, RAD21, EP300, MPL, SBDS, NF1). Over a median follow-up of 48 months (6-278), 27% of patients, younger and with higher PLT counts (Table1) were judged eligible to observation-only whilst the others received CyA (42%), CyA+eltrombopag (10%), eltrombopag alone (6%), and other treatments (15%; N=16 androgens, 8 ATG, 3 alemtuzumab, 2 daclizumab, 1 azathioprine, and 5 unknown). The overall response rate (CR+PR) at 6 months in evaluable patients (N=144) was 76% for CyA, 56% for CyA+ eltrombopag, 54% for eltrombopag, and 61% for other, without significant differences across treatment groups. The distribution of PLT, Hb, and ANC responses are detailed in Table 1, with 22% achieving a bilineage and 34% a trilineage response. Approximately 15% of cases experienced >1 adverse event (AE) of grade >2. Grade 3/4 toxicities were more common in the CyA+eltrombopag group and encompassed retinal thrombosis, gastroenteritis, diarrhoea and transaminitis. Therapy discontinuation occurred in 17% of treated subjects due to persistent CR (6), non response (5), AE (14), and death (5), equally distributed between CyA and CyA+eltrombopag group. Sixty-three patients died during the follow-up with infections as the leading mortality cause. Poorer survival associated with older age [HR 1.24 (95% CI 1.1-1.4), p<0.001], PLT and RBC transfusion dependence [5.2 (1.8-15), p=0.002; and 3.56 (1.4-9.1), p=0.008, respectively], and presence of reticulin fibrosis [3.2 (1.4-7.4), p=0.007] at baseline.
This is one of the largest NSAA series ever reported highlighting that the use of CyA+/- eltrombopag leads to a significant clinical improvement with a favourable impact on survival. Occurrence of G3 gastrointestinal/liver toxicity in patients receiving combination therapy deserves attention and further studies.
Disclosures
Fattizzo:Momenta: Consultancy; Janssen: Consultancy; Amgen: Consultancy; Sobi: Speakers Bureau; Alexion: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau. Gandhi:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Alexion, AstraZeneca Rare Disease: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Jazz: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Sobi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Maciejewski:Alexion: Consultancy; Apellis Pharmaceuticals: Consultancy. Kulasekararaj:Pfizer: Consultancy, Honoraria, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau; Sobi: Consultancy, Honoraria, Speakers Bureau; Samsung: Consultancy, Honoraria, Speakers Bureau; F. Hoffmann-La Roche: Consultancy, Honoraria, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Biocryst: Consultancy, Honoraria, Speakers Bureau; Apellis: Consultancy, Honoraria, Speakers Bureau; Amgen: Consultancy, Honoraria, Speakers Bureau; Alexion, AstraZeneca Rare Disease: Consultancy, Honoraria, Speakers Bureau; Akari: Consultancy, Honoraria, Speakers Bureau; Achillion: Consultancy, Honoraria, Speakers Bureau; Novo Nordisk: Consultancy, Honoraria, Speakers Bureau; Ra Pharma: Consultancy, Honoraria, Speakers Bureau. Barcellini:Momenta: Honoraria; Incyte: Membership on an entity's Board of Directors or advisory committees; SOBI: Honoraria; Janssen: Honoraria; Biocryst: Honoraria; Apellis: Honoraria; Alexion: Honoraria; Bioverativ: Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria; Agios: Honoraria, Research Funding; Sanofi: Honoraria, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal